Nearly 30,000 healthy volunteers in more than 140 countries have signed up for the “Human Challenge trial” to help scientists speed up the vaccine clinical trials (Healthline). In human challenge trials, healthy people are given a potential vaccine and then infected with the virus intentionally. Though the trial raises many ethical questions, currently there seems to be no better option than human challenge trials for researchers and scientists to expedite the development process of a potential vaccine or drug against the COVID-19 virus.
Originated in Wuhan, China, the COVID-19 epidemic transformed into a pandemic as it started spreading at an alarming rate across various geographies of the world. Today the number of confirmed COVID-19 cases has surpassed 9 million and the number is still increasing, despite the proactive measures like nationwide lockdown and self-isolation protocols taken to fence the spread of the deadly contagion.

People have confined themselves to the walls of their houses and have been living a new-normal since the start of the year, 2020. And throughout this period, the only momentous question on everyone’s mind has been– when will we get a vaccine or drug for COVID-19?
This article gives an overall picture of the drug and vaccine development process since the start of the COVID-19 pandemic.
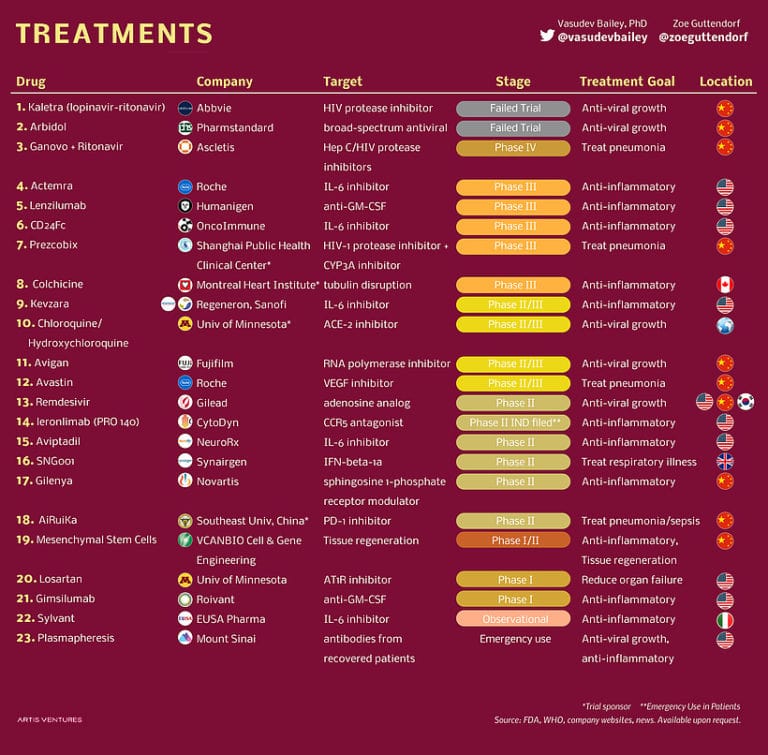
Anti-viral drugs tested against COVID-19 so far
Since the development of vaccines typically takes a longer time than anti-viral drugs, many companies have been working on the development of anti-viral drugs to treat people infected with the Novel Coronavirus. Following are some of the anti-viral drugs that were used to combat COVID-19:
Remdesivir: This drug was developed a decade ago and was tested against the Ebola virus in 2014. Though found to be safe in people, the drug failed in the clinical trials against Ebola. However, the drug when tested against MERS (a disease caused by a different member of the Coronavirus family), blocked the replication of the virus. Hence, it is now tested against COVID-19 in many clinical trials across the globe.
According to Dr.Anthony Fauci, director of NIAID (National Institute of Allergy and Infectious Diseases) Remdesivir reduced the recovery time of infected patients. However, another study published in The Lancet report, stated that the results of clinical trial subjects who took Remdesivir weren’t different from the people who took a placebo.
On May 1, despite the conflicting results, the FDA issued an emergency use of Remdesivir.
Arbidol: This anti-viral drug was tested in combination with Ritonavir/Lopinavir in people with mild to moderate COVID-19 infection. However, in mid-April, researchers announced the two drugs didn’t produce any positive clinical outcomes.
Source: Ritholtz
EIDD-2801: Created by a group of scientists at a non-profit biotech company owned by Emory University, the drug, when tested in mice, blocked the replication of multiple Coronaviruses, including COVID-19. As a consequence of this, in May, the pharmaceutical company Merck and Ridgeback Biotherapeutics LP signed an agreement for the development of this drug. The drug is already under clinical trials in the UK.
Favipiravir: Approved to treat influenza in some countries outside the US, this drug was suggested as an effective treatment for the Novel Coronavirus in some reports from China. Japan, the prime manufacturer of Favipiravir, is currently distributing the drug to 43 countries across the globe for clinical trials in patients with mild to moderate COVID-19 infection. Canadian researchers are also testing the drug for long-term results.
Kaletra: This is a combination of two drugs: Lopinavir and Ritonavir. Kaletra is currently under clinical trials against SARS-CoV-2 (COVID-19). According to a study published by Cell Press on May 4, neither Lopinavir nor Ritonavir produced positive outcomes when tested against mild or moderate COVID-19 cases. This fact was backed by another study published on May 7, in the New England Journal of Medicine.
However, according to another study published by Lancet on May 8, the drug Lopinavir/Ritonavir when tested along with Ribavirin and Interferon beta-1b, seemed to clear the virus from people’s body in less time.
Merimepodib: Developed by ViralClear Pharmaceuticals Inc., this drug was tested against Hepatitis C previously. Now the company is conducting the phase-2 trial of this drug against COVID-19. People who are in the advanced stages of COVID-19 will be picked randomly to receive either one of the following combinations: Merimepodib + Remdesivir, Remdesivir + placebo. The company hopes to have the trial result by the late summer of 2020.
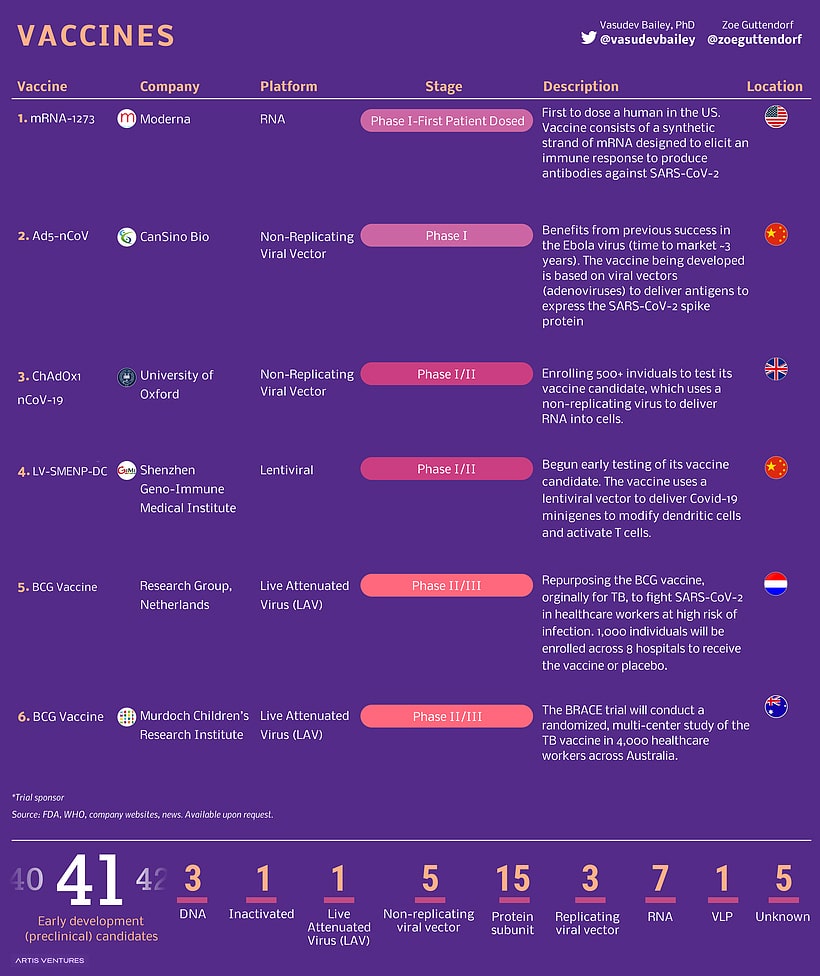
List of vaccine development projects
Generally, a vaccine is created to prevent people from getting infected from a virus. Or in other words, a vaccine actually trains the immune system to recognize and attack a virus when encountered. Besides the vaccinated person, the community around the person is also protected from the virus with a vaccine. To give a clearer context, when a person is vaccinated, he/she cannot be infected with the virus, which also means the person cannot pass the virus to other people. In clinical terms, this is known as Herd Immunity. Hence the importance of vaccine development outweighs that of drug development.
Below are some of the vaccine development projects for COVID-19:
Moderna: The company developed a vaccine called messenger RNA or mRNA and started with the phase-1 clinical trial in March, in Seattle, Washington. Then, in mid-May, the company reported that the vaccine produced antibodies in all the 45 test subjects, in the initial stage of clinical trials. The test subjects were from 18 to 55 years of age and received two shots 28 days apart.
Following the positive results from the first stage of clinical trials, the company sought FDA’s permission for the second phase of clinical trials. Moderna successfully completed the phase-2 trials and started with phase-3 trials of their vaccine in late July.
Inovio: The company was already working on a DNA vaccine for MERS (Middle East Respiratory Syndrome – related to Coronavirus) when the COVID-19 pandemic was spreading all over the globe. Hence, the company was able to develop a potential vaccine for COVID-19 quickly. At the end of April, the company announced that it has enrolled 40 healthy volunteers for the first stage of the clinical trials. The secondary phases of the clinical trial were planned for summer.
Source: Onesha
Bharat Biotech: An Indian biotechnology company headquarted in Hyderabad, in collaboration with Indian Council of Medical Research (ICMR) has developed a COVID-19 vaccine called COVAXIN. Approved by DCGI (Drug Controller General of India), the vaccine is likely to undergo phasse-1 and phase-2 of human clinical trials from July 2020.
University of Oxford in England: In late April, Oxford officials started a clinical trial with more than 500 participants and claimed the developed vaccine has an 80% chance for success. They even announced that if the clinical trial was successful, they could deliver at least 30 million vaccine doses as early as September.
The university collaborated with the pharmaceutical company, AstraZeneca, and developed a vaccine that triggers the immune system with a modified virus. In mid-May, the company stated that the vaccine was effective in Rhesus Macaque monkeys infected with COVID-19 and plans to begin a final-stage clinical trial by the middle of this year.
Serum Institute of India (SII): The Pune-based manufacturer of vaccines and immuno-biologicals, SII, partnered with the biopharmaceutical company AstraZeneca, to manufacture the experimental COVID-19 vaccine developed by University of Oxford. The company’s CEO announced that, if trials are successful, the vaccine will be launched under the name ‘Covishield’ in the UK and India. In fact, the company has planned to produce 3 to 4 million doses of the experimental COVID-19 by the end of 2020 (Hindustan Times).
University of Queensland in Australia: Researchers are growing viral proteins in cell cultures to develop a vaccine for novel coronavirus. Following the preclinical testing conducted in early April, phase 1 trial on human subjects began in early July.
Pharmaceutical companies: Some of the pharmaceutical companies are also working on their own COVID-19 vaccine development projects. For instance, Johnson & Johnson is working in association with Sanofi to formulate a vaccine for the deadly contagion. In fact, Johnson & Johnson announced publicly that they will begin first stage clinical trials in July.
Sinophram- a China-based pharmaceutical company has developed a vaccine for COVID-19 and is the world’s first to enter the phase-3 clinical trial. The trial is conducted in association with Abu Dhabi Goverment and an Abu Dhabi-based AI company called G42 healthcare.
Similarly, Pfizer has joined hands with a German biotech company called BioNTech to develop a vaccine. According to Pfizer’s announcement in early July, the vaccine created an immune response on test subjects during the early stage of the clinical trial, but caused side effects like fever at high dosages.
Will there be a potential vaccine or drug for COVID-19 by the end of 2020?
The advancements in technology have aided greatly in treating and developing new treatments for the Novel Coronavirus. However, deciding a timeframe for vaccine or drug development is not an easy task as the process includes laboratory testing, animal testing, and various stages of clinical trials. Additionally, scientists have to wait and observe the side effects of the drug, before getting it approved for people’s use.
Though some experts have stated that the timeline might extend up to summer or fall 2021, up until now, no company has given a clear timeline for a potential vaccine or drug against the Novel Coronavirus.